Investigational IVD research sponsors (and sponsor-investigators) need to make SR/NSR device determinations and provide study-specific justification for their assessment to institutional review boards (IRBs). Clinical trials involving investigational in vitro diagnostic (IVD) devices are subject to the Food and Drug Administration’s (FDA’s) investigational device exemption (IDE) requirements (21 CFR 812). Unless the study meets certain IDE exemption criteria, sponsors (or sponsor-investigators) must determine whether using an investigational IVD presents:
- Significant risk (SR) and is subject to the full IDE requirements, or
- Nonsignificant risk (NSR) and is subject to the abbreviated IDE requirements.
Significant Risk vs. Nonsignificant Risk:
A clinical trial is deemed SR when it involves an investigational medical device presenting a potential for serious risk to the participant’s health, safety, or welfare. When an investigational device does not carry this potential for serious risk, the clinical investigation is NSR. This FDA page describes SR/NSR determinations.
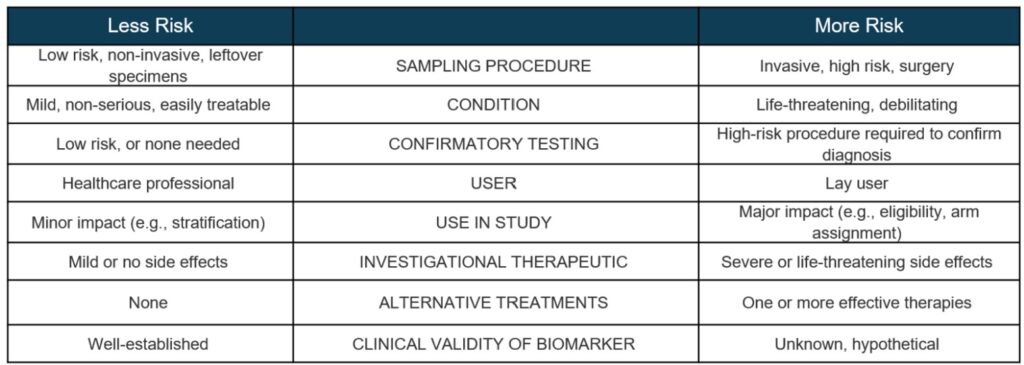
In IVD research, device risk determinations are primarily tied to sampling procedures and the consequences of invalid test results. False positives or negatives may lead to a misdiagnosis or mismanagement of a participant’s condition.
Sampling Procedures:
In some cases, an investigational IVD will require research participants to undergo high-risk sampling procedures that are not standard of care. In these situations, the use of the IVD is SR and requires an Investigational Device Exemption (IDE).
Sampling procedures presenting SR include:
- Biopsy of a major organ;
- Sampling requiring general anesthesia or prolonging standard of care surgery;
- Placement of a blood access line into an artery or large vein.
When an investigational IVD requires any of these above procedures for research purposes, the study is usually SR.
Conversely, NSR procedures are typically:
- Blood obtained through finger stick or simple venipuncture;
- Saliva collection, buccal swabs, and other non-invasive sampling;
- Skin punch biopsies;
- Biopsy of the upper gastrointestinal tract using endoscopy;
- Use of existing specimens, such as archival tumor tissue or samples from biorepositories;
- Use of otherwise-discarded remnant tissue from standard of care surgical procedures.
Although these procedures are usually NSR, researchers should also consider the characteristics of the clinical trial population. Their current health status, comorbidities, and/or medications could raise the risks of certain sampling procedures to SR.
Regardless of how the IVD results are used in the study, if SR sampling procedures are used, the clinical trial is SR.
Impact of Invalid Test Results:
If the research does not involve SR sampling, the next step is to assess the nature of potential harm to participants from inaccurate test results.
All investigational IVD clinical trials should consider the impact returning results could have on participants’ clinical care outside of the trial. False positive results could lead to misdiagnosis, ineffective treatments, or unneeded confirmatory testing. The greater the risks associated with unnecessary clinical care, the greater the risk of the investigational IVD.
False positives can also cause psychological trauma if a participant erroneously believes they have tested positive for a serious disease.
False negative results, on the other hand, might lead participants to forego or delay needed treatment, which could have serious implications for their health.
As part of this assessment, researchers need to consider how IVD results will be returned to participants and/or their healthcare providers. IVDs intended for home use may present greater risk than a lab-based test because a lay user (rather than a trained healthcare professional) will interpret the results.
Considerations for Investigational IVDs Used in Therapeutic Product Trials:
Clinical trials of therapeutic products (like drugs or biologics) should take into consideration additional protections when:
- They involve co-development of a companion diagnostic, or
- They use an investigational IVD to select participants, assign treatment arms, monitor safety, or predict adverse outcomes.
In such studies, the therapeutic agent is a larger factor in the SR/NSR determination. This is because the safety and effectiveness of the therapeutic agent hasn’t been fully established.
Additional protection considerations sponsors might include when making device risk determinations:
Will using the results from an investigational IVD lead to some study participants foregoing or delaying a treatment known to be effective?
Consider treatments available to participants outside of the clinical trial. The risks of false positive results from an investigational IVD are lower when the study will only enroll individuals who have already exhausted all approved treatments for their condition.
The risk of invalid results increase when there are well-established treatments available outside of the clinical trial. In this case, researchers should consider more than just the risks of foregoing or delaying established treatment. Also critical is determining whether receiving an investigational drug could reduce the participants’ suitability for, or the effectiveness of, existing therapies after the study concludes.
Will use of the results from an investigational IVD expose study participants to safety risks (e.g., adverse events from the investigational therapeutic product) that exceed the risks encountered in non-trial standard of care or the control arm therapy?
An investigational IVD’s risk assessment must include the potential side effects of the investigational treatment administered in the clinical trial.
As the severity of side effects increases, so does the health impact of invalid IVD results – which pushes the use towards SR.
Is it likely, based on existing knowledge about the relationship between the biomarker and the investigational therapeutic product, incorrect results from the investigational IVD would present a potential for serious risk to study participants?
Consider not only the analytical validity, but also the clinical validity of an investigational IVD.
For example, a study drug may be hypothesized as more effective in individuals with a certain mutation, but the link is not well-established. Using an investigational IVD to identify and enroll only those with the mutation of interest raises the overall risk of the IVD.
Further Assistance with IVD Risk Determinations:
Sponsors and sponsor-investigators of clinical trials involving investigational IVDs should address these factors when crafting their SR/NSR assessment. If the IRB disagrees with your determination, they will provide a rationale based on the factors discussed above.
The FDA also welcomes queries regarding device risk determinations. The traditional way of obtaining a formal device risk determination is through the Q-Submission process. For clinical trials of therapeutic products conducted under an IND, sponsors can contact their program officer for help determining if an investigational IVD is SR and requires an IDE submission.
Finally, it is important for both researchers and IRBs to understand device risk determinations are unique to each study. The determinations may need reassessment over the study’s lifetime if the investigational IVD use changes.
Assessing Risk for Non-IDE Exempt IVD Investigations: